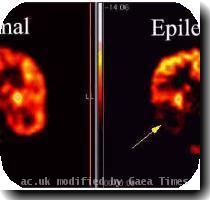
FDA panel narrowly favors approval for Medtronic implant to treat epilepsy
By APFriday, March 12, 2010
FDA panel backs Medtronic brain stimulator
WASHINGTON — Federal health advisers say a brain stimulating device from Medtronic can be approved for epilespy, provided the company conducts follow-up safety studies.
The Food and Drug Administration’s panel of neurological experts voted 7-5 in favor of Medtronic’s Deep Brain Stimulation implant, under certain conditions.
The experts said it should carry warning about depression and memory loss. They also said Medtronic should track patient safety for five years
The FDA is not required to follow the panel’s advice, though it usually does.
Medtronic has asked the FDA to approve its implant for reducing seizures caused by epilepsy, a neurological disease.
The device is already approved for other movement disorders, including Parkinson’s Disease.
|
March 15, 2010: 12:54 pm
A loan will look better on your Bureau and will also give you the added bonus of keeping the room on your credit cards open. |

dental implants