Mixed reviews for experimental pills to treat common form of multiple sclerosis
By Mike Stobbe, APWednesday, January 20, 2010
MS pills show promise and risk, studies say
ATLANTA — Tests of the first two oral drugs developed for treating multiple sclerosis show that both cut the frequency of relapses and may slow progression of the disease, but with side effects that could pose a tough decision for patients.
Two experts not involved in the studies said the drugs appear effective but with potentially dangerous side effects. It’s too soon to know if the pills will be approved by the government or widely adopted by physicians, they said.
About 2.5 million people around the world have multiple sclerosis, a neurological disease that can cause muscle tremors, paralysis and problems with speech, memory and concentration. The studies involve the most common form of the disease, in which people are well for a while and then suffer periodic relapses.
Current treatments can reduce the duration and severity of symptoms but require daily or regular shots or infusions.
The new studies tested two types of pills. Cladribine, made by Merck Serono, is already sold to treat a rare blood cancer. For MS, it would be taken eight to 10 days a year. Fingolimod is a daily MS pill being developed by Novartis.
The research found that patients on the pills were about half as likely to suffer relapses of symptoms as those who took dummy pills or a commonly prescribed shot for MS.
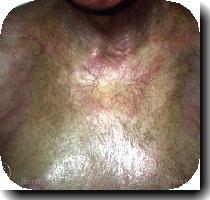
But they also found both drugs significantly lowered immune defenses that allowed latent herpes viruses to rage in some patients — in one study, two people died of unchecked herpes infections.
The side effects detailed in the new studies are giving some physicians pause.
“There is a price tag attached” to the new medications, said Dr. Silva Markovic-Plese, an MS researcher at the University of North Carolina.
The studies were being published in Thursday’s New England Journal of Medicine.
There is no cure for MS, but steroids can reduce the duration and severity of symptoms in the short term, and seven treatments on the market have had success in reducing recurrence of symptoms.
All involve daily or regular injections. So a pill is appealing: Even healthy people can have trouble giving themselves shots, so it can be a nightmare for patients with faltering coordination and concentration.
“Right now I have to think very hard when I make coffee,” said Ivana Vuletic, a 49-year-old Chapel Hill, N.C. woman with MS. “I would be greatly relieved if I didn’t have to prick myself or be pricked” with needles, she said.
Still, she said she wouldn’t take the new pills if their side effects were too dangerous.
Vuletic is on her third MS drug in about four years, and her illness has gotten worse. The MS injections have led to pocked skin and other problems so she is leery of new treatments’ side effects.
The new studies reveal the trade-offs:
—A two-year study gave 1,300 MS patients cladribine or dummy pills. Patients on the drug were only half as likely to suffer relapse as those on placebo, and were 30 percent less likely to have worsening disability. However, 20 percent to 30 percent of the cladribine patients developed low counts of infection-fighting white blood cells, compared to just 2 percent of the others. And 20 cladribine patients suffered herpes infections versus none in the dummy pill group.
—A two-year study gave about 1,000 patients fingolimod or dummy pills. Only 17 percent of fingolimod patients had worsening disabilities from MS after three months, compared to 24 percent in those on placebo. Herpes infections were about the same in the pill and placebo groups, but respiratory infections like bronchitis and pneumonia were nearly twice as common in the fingolimod patients.
—A one-year study of 1,200 patients tested fingolimod against shots of Avonex, a form of interferon. Those taking the pills had less brain shrinkage — a measure of progression of the disease. About 20 percent of patients on the pill had relapses versus 30 percent on the dummy pills.
In that study, 9 percent of those on fingolimod had serious side effects, compared to 6 percent of those on Avonex. Two people on fingolimod died of herpes infections; six had eye swelling and eight had skin cancers.
All three studies were funded by Novartis or Merck Serono, the pill manufacturers.
Doctors are likely to turn first to current options until the pills’ side effects are better understood, said Dr. Neil Lava, the director of Emory University’s multiple sclerosis clinic.
Physicians are mindful of what happened with Tysabri, an MS drug that was approved in November 2004 and pulled from the market the next year after cases of a rare but lethal brain inflammation in some patients. It was reintroduced in 2006, but doctors are still monitoring for side effects, Lava said.
Tags: Atlanta, Diagnosis And Treatment, Diseases And Conditions, Georgia, Medication, Multiple Sclerosis, North America, United States
|
January 21, 2010: 6:15 am
I have seen your post and this post is very useful for us.I have read your all concepts that seems useful for us. |
JMueller