New vaccine offers chance for children in Africa, where 1 million die of malaria each year
By Jason Straziuso, APTuesday, November 3, 2009
New vaccine offers hope in Africa’s malaria battle
SIAYA, Kenya — A mother watched with dread as a nurse inserted a tube in her baby’s head. Blood streamed into the anemic 4-month-old who already has malaria, the mosquito-borne disease that kills a million African children every year.
“Malaria is one of the deadliest sicknesses for children,” the nurse said — words that sent the young mother into a crumpled heap on the bed beside her wide-eyed baby boy, wrapped in a blue-and-yellow floral blanket.
There is new hope, however, in this verdant area where President Barack Obama’s relatives live. A vaccine that appears to be able to prevent the disease in about 50 percent of children, is now undergoing the final stage of testing.
If regulators determine the vaccine is safe, it could be on the market in three to five years — the first vaccine against a human parasite.
Tens of millions of Africans are plagued by malaria every year, and more than a third of the hospital beds in this rural Kenyan region next to Lake Victoria are dedicated to its victims. More than 1 million children die of the disease in Africa annually, a crippling economic drain that prolongs a cycle of disease and poverty throughout the continent.
Malaria is also prevalent in parts of Asia, the Middle East and Central and South America.
This vaccine was developed specifically for Africa, however, and will only prevent the African strain of the disease. Experts say it would be a historic advancement.
“Some may say, ‘50 percent, that’s not great.’ And that’s true. If you get a measles vaccine, you’re not going to get measles again,” said Dr. Dave Jones, a U.S. Army colonel and director of a clinic in nearby Kombewa operated by the Walter Reed Army Institute of Research and the Kenya Medical Research Institute.
“But at the same time, when you consider we lose 1 million kids a year, if you could cut that in half it would be a great step forward.”
Experts from around the globe are meeting in the Kenyan capital, Nairobi, this week as part of the fifth pan-African malaria conference, and a news conference on the vaccine trial is scheduled for Tuesday.
More than $500 million has been spent on the combined efforts by drug maker GlaxoSmithKline and the PATH Malaria Vaccine Initiative, which is funded by the Bill & Melinda Gates Foundation. The Phase III testing is being done at 11 sites in seven African countries on 16,000 children under the age of 18 months.
The goal is to immunize children against malaria during their youngest high-risk years, and then for them to develop their own natural immunities as they age.
At the spartan, open-air clinic in Kombewa last week, Patrician Mrunde, a 34-year-old mother of six, sat in the hallway with her youngest, 6-month-old Linda, who was waiting to receive a shot as part of the trial.
Mrunde has seen her eldest child stricken with fever and lapse into convulsions from malaria, and a young relative die from it.
“I decided to join the study to get help for the disease,” she said.
Dr. Allen Otieno, a 38-year-old pediatrician, said “everybody is afraid” of malaria in the region. He called the new vaccine promising. “As scientists we have great hope that it will reduce the burden of malaria,” he said.
Joe Cohen, a top researcher for GlaxoSmithKline, said all the data collected during testing have been encouraging.
The 66-year-old Cohen, who has been working on a malaria vaccine for two decades, said the trial results will be submitted to regulators in 2012, and that a vaccine could be on the market shortly afterward.
No prices have been set for the vaccine, Cohen said, though families in Africa may not have to pay anything for it because the Gates Foundation, UNICEF, WHO and the GAVI Alliance would provide funds.
GlaxoSmithKline “is committed to making sure pricing will never be a barrier to access for this vaccine,” Cohen said.
The vaccine has been in development for more than 20 years through the combined efforts of GlaxoSmithKline, the Malaria Vaccine Initiative, the Walter Reed Army Institute of Research and others.
“No single person could have ever achieved this,” Cohen said. “That’s the lesson that should be taken out of the collaboration.”
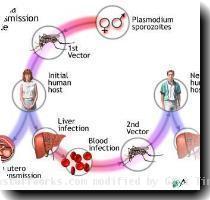
Malaria is caused by a parasite and spreads through a bite from an infected mosquito. The parasite travels quickly to the liver where it matures, enters the bloodstream and causes fever, chills, flu-like symptoms and anemia. The vaccine is designed to attack the parasite before it can infect the liver.
Until now, the main line of defense in preventing the disease has been distribution of bed nets and mosquito spraying.
Jonathan Odro Anyumba, chairman of the board of the Kombewa district hospital, said malaria is a huge burden in this verdant area of Kenya, where many live in mud huts and collect water in plastic jugs from flowing streams.
Families must sleep under nets to protect against the disease, though many don’t have any. Even half the beds at his hospital don’t have nets, Anyumba said.
“When you visit these areas you’ll find that each and every child has malaria. Thirty to 50 percent of the deaths in this community are from malaria,” he said. “I think this vaccine is going to be very, very useful.”
On the Net:
PATH Malaria Vaccine Initiative, www.malariavaccine.org
GlaxoSmithKline, www.gsk.com/malaria
Tags: Africa, Animals, Arthropods, Child And Teen Health, Diseases And Conditions, East Africa, Health Care Industry, Immunizations, Infectious Diseases, Kenya, Medical Research, Public Health, Siaya